Christine Frayret
Université de Picardie Jules Verne, France
Title: Rational design of organic electrode materials: New advances and tools
Biography
Biography: Christine Frayret
Abstract
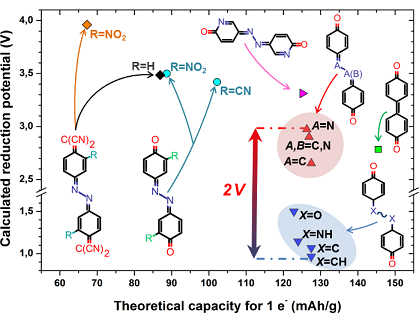
The demand for sustainable energy sources has stimulated great interest in new technologies and materials. A multitude of research efforts in recent years have led to marked progress in this area, including in particular lithium-ion batteries based on organic electrodes. A significant milestone in designing such materials would correspond to the comprehension of the rules governing the increase/decrease of the redox potential of molecular entities, especially if differentiation between various backbones/functional groups/redox centers or incidence of atom substitution within rings can be reached. Based on the combined use of molecular quantum simulation and accurate examination of the electronic structure properties, a rational design of organic electrode materials can be performed. In addition to spin density populations’ estimation, different additional tools can be used to understand molecular redox potential trends. For instance, NICS (nuclear independent chemical shift), HOMA (harmonic oscillator model of aromaticity), d FLU indicators, etc., have the opportunity to electron delocalization on various rings/bond paths within a molecule in a quantitative way. In this context, the partitioning of the global energy of the molecule may constitute another analysis instrument to shed light on the various pieces of molecule playing the main role in stabilization/destabilization upon reduction or how balance between some competitive effects does occur. Such approach can be applied to systematic theoretical screening of existing compounds/hypothetical novel candidates, both being tuned through various effects. This should progressively complete the database for the discovery of new/optimized organic electrodes with improved features. Thanks to this methodology a work of prospection has been undertaken on various families, including derivatives of quinone, carboxylate, quinoneazine and pentalenedione. By focusing on these various sets of compounds, we identified some property-based guidelines, which may serve not only for the ranking of the studied entities but also for the search of more advanced systems.