
Hikaru Kobayashi
Osaka University, Japan
Title: Si nanopowder for internal hydrogen generation materials
Biography
Biography: Hikaru Kobayashi
Abstract
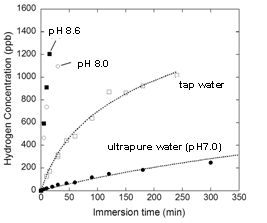
Although Si bulk doesn’t strongly react with water, Si nanopowder reacts with it when its size is less than ~20 nm, leading to generation of hydrogen. In previous literature, reactions of Si nanopowder with strong alkaline solutions have been investigated to achieve high hydrogen generation rates for application to e.g., fuel cells. In the present study, we have aimed at generation of hydrogen in internal conditions. Hydrogen generated in the body, especially in bowels, is effectively absorbed, is circulated, and reacts with hydroxyl radicals which cause various diseases such as cancer, Alzheimer’s disease, Parkinson’s disease, etc. Figure 1 shows the concentration of hydrogen generated by the reaction of 1 g Si nanopowder with water in the neutral pH region. Hydrogen was generated even with ultrapure water, but the generation rate was very low. The hydrogen generation rate greatly increased with pH while pH didn’t change after the hydrogen generation reaction. Therefore, the reaction schemes are written as:
Si+2OH-→SiO2+H2+2e, (1)
2H2O+2e→H2+2OH-. (2)
In the initial reaction, Si reacts with OHï¼ ions to generate hydrogen, SiO2, and electrons most probably in the SiO2 conduction band. In the subsequent reaction, generated electrons are accepted by water molecules, resulting in formation of hydrogen and OHï¼ ions. Reaction (1) is the rate-determining step, and thus, the reaction rate greatly increases with pH. The above result indicates that when Si nanopowder is taken, it doesn’t react in stomach under acidic conditions due to gastric acid, but reacts with water in bowels in alkaline conditions because of pancreatic juice. We have performed hydrogen generation experiments under conditions similar to bowels, i.e., pH 8.3 and 36ºC. In this case, more than 300 mL hydrogen was generated from 1g Si for 20 h. This hydrogen volume corresponds to that contained in more than 17 L saturated hydrogen-rich water.
Recent Publications:
- Imamura K, Irishika D, Kobayashi H, (2017) Mechanism of ultra-low reflectivity for nanocrystalline Si/crystalline Si structure formed by surface structure chemical transfer method. J. Appl. Phys. 121: 013107-1-5.
- Imamura K, Kimura K, Fujie S, Kobayashi H, (2016) Hydrogen generation from water using Si nanopowder fabricated from Si swarf. J. Nanopart. Res. 18: 116-1-7.
- Matsumoto T, Maeda M, Kobayashi H (2016) Photoluminescence enhancement of adsorbed species on Si. Nanoscale Res. Lett. 11: 7-1-6.
- Matsumoto T, Maeda M, Furukawa J, Kim W B, Kobayashi H (2014) Si nanoparticles fabricated from Si swarf by photochemical method. J. Nanopart. Res. 16: 2240-1-7.


