
Azwani Sofia Ahmad Khiar
Universiti Sains Islam, Malaysia
Title: Conductivity Studies of Methyl Cellulose/Chitosan/1-butyl-3-methyl imidazolium bis(trifluoro sulfonyl) imide Doped With Ammonium Triflate Based Polymer Electrolyte
Biography
Biography: Azwani Sofia Ahmad Khiar
Abstract
Statement of the Problem: Polymer electrolyte is an ionic conductor containing inorganic salt dissolved in polymer host. Among other, liquid-based electrolyte are commonly used in electrochemical devices due to its high conductivity. Nevertheless, some drawbacks of liquid electrolyte could cause safety and environmental issues. Hence, solid biopolymer electrolyte (SBE) are proposed to overcome the problems. However, one drawback is the low conductivity of SBE has hindered their applications. Thus, this work was carried out to enhance the conductivity of SBE based on methylcellulose/chitosan blend doped with 1-butyl-3-methyl imidazolium bis (trifluoro sulfonyl) imide (BMIMTFSI) ionic liquid and ammonium triflate (NH4TF) salt.
Material and Method: A mixture of MC/CS/45 wt% BMIMTFSI containing different weight percentage of NH4TF was prepared by using solution casting technique. Electrochemical Impedance Spectroscopy (EIS) was used to measure ionic conductivity over a wide range of frequency between 50Hz-1MHz and at temperatures between 298 K and 378 K.
Findings: Maximum conductivity achieved is 7.67 x 10-4 Scm-1with 25 wt% of NH4TF at ambient temperature. Dielectric data shows that the increase in conductivity could be due to the increase in number of charge carriers while modulus study confirms the non-Debye behavior. Conductivity study at elevated temperatures suggest that samples are Arrhenius in nature.
Conclusion & Significance: MC/CS/45 wt% BMIMTFSI /25 wt% NH4TF polymer electrolyte was successfully prepared. The sample achieve a maximum conductivity of 7.67 x 10-4 Scm-1 at ambient. The increase in conductivity could be attributed to increase in mobile ions while the decrease could be attributed to ion association. Temperature helps to assist ion movement and provide alternative pathway for the cation hoping, hence boost the conductivity.
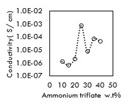

Figure 1: Conductivity plot as a function Figure 2: Log conductivity
Versus of salt content 1000/T
Recent Publications
1. Chinnam, P. R., Hanjun Zheng, Stephanie L. Wunder. (2015). Blends of Pegylated Polyoctahedralsilsesquioxanes (POSS-PEG) and Methyl Cellulose as Solid Polymer Electrolytes for Lithium Batteries. Electrochim Acta. 170:191–201.
2. Radzir N.N.M. (2015). An investigation of polymer electrolyte based on poly (glycidyl methacrylate) doped withimidazolium ionic liquid. J. Mater. Environ. Sci. 6:1436-1443.
3. Ramlli M. A. & M. I. N. Isa. (2015). Structural and ionic transport properties of protonic conducting solid biopolymer electrolytes based on carboxymethyl cellulose doped with ammonium fluoride. J. Phys. Chem. B5: 748 – 752.
4. Kadir M. F. Z. (2017). Biopolymeric electrolyte based on glycerolized methyl cellulose with NH4Br as proton source and potential application in EDLC. Ionics. https://doi.org/10.1007/ s11581-017-2330-4.
5. Ariffin N. A., & A. S. A. Khiar. Effect (2015) of BMITFSI to the electrical properties of Methylcellulose/chitosan/NH4TF based polymer electrolyte. Proc. of SPIE 2015; 9668: 96681J.

