Haruhiko Morito
Tohoku University, Japan
Title: Crystal growth of Si based on the Na-Si binary phase diagram
Biography
Biography: Haruhiko Morito
Abstract
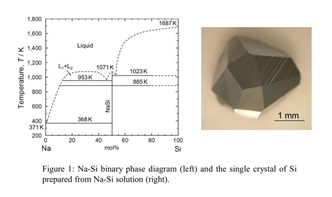
Phase diagram provide essential information for the conditions of materials synthesis and crystal growth. Although many binary phase diagrams were reported in the last century, that for Sodium (Na) and Silicon (Si) has not yet been established. In 2009, our group has presented a Na–Si binary phase diagram with the results of thermal analyses and morphology observation. In the present study, we demonstrated the crystal growth of Si from the Na–Si solution based on the Na–Si phase diagram. As shown in the Na-Si binary phase diagram (Fig. 1), Si is dissolved in a Na melt at 1173 K. Since the boiling point of Na is 1154 K at 1 atm and the vapor pressure of Na is relatively high above 973 K, Na can be removed from the products by evaporation. The Na-Si mixture (molar ratio Na/Si = 3:2) was heated at 1173 K. Na evaporation changed the composition of the sample toward the liquidus line at around 55 mol% Si at 1173 K, allowing crystallization of supersaturated Si to begin. After Na evaporation, single crystal of Si was obtained as shown in Fig. 1. Likewise, various Si crystals such as Si film, porous bulk Si and Si micro-tube were prepared by using a Na-Si solution. Furthermore, the efficient removal of impurities in Si for the solar cell was demonstrated by dissolution and recrystallization in a Na melt at low temperature. Recently, we succeeded in the crystal growth of Si clathrates by using a Na-Sn flux. These compounds have been widely studied due to their unique open-framework structures of Si polyhedrons.