Christelle Delaite
LPIM - Université de Haute-Alsace, France
Title: MSE-16: Random poly (É›-Caprolactone-L-Alanine) by direct melt copolymerization
Biography
Biography: Christelle Delaite
Abstract
During recent years, many research works have been directed to the preparation of biodegradable and biocompatible polymeric materials with controlled chemical, physical, and biological properties for a wide range of biomedical applications in the fields of surgical implants, surgical sutures, artificial skin, resorbable bone plates, tissue engineering scaffolds and carrier system for controlled release of drogue and genes.
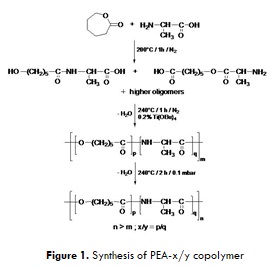
Various synthetic strategies can be used for the preparation of amino acid-based poly(ester amide)s (AA-PEAs)presenting block, alternating or random structures. Random PEAs can be prepared by simple procedures that do not require the use of solvents or expensive monomers like α-amino acid N-carboxyanhydrides (NCAs). Some studies have reported the synthesis of Random AA–PEAs by the direct reaction of amino acids with cyclic esters or by the direct bulk polycondensation of amino acids and α-hydroxyacids.
The aim of the present work is to study the synthesis and properties of random polyesteramides prepared by the bulk copolymerization of inexpensive É›-caprolactone and L-alanine, using a simple one-step procedure. A series of random polyesteramideswithin a range of molar composition from 90/10 to 50/50 were synthesized by a direct melt polycondensation. Their structure was fully characterized by FTIR and NMR spectroscopy. The resulting copolymers are completely amorphous with the exception of PEA-90/10 which possess a semi-crystalline structure. These PEAs present increasing glass transition temperatures at increasing L-alanine contents, and exhibit fairly good thermal stability with 10 % mass loss temperatures reaching 315°C.